Abstract
Biomolecule-based nanostructures are of great interests in bio-nanomaterial design due to their rich structural diversity and inherent biocompatibility. Among them, cyclic peptides have received remarkable attention owing to their rigid configuration as well as chemical and enzymatic stability. To date, previous reports have mainly focused on the construction of nanotubes in a ring-stacking manner through hydrogen bonding. However, other assembling strategies have rarely been explored. In this work, two cyclic peptides, cyclo-(DP)3 and cyclo-(DG)3, have been rationally designed and used as building blocks to fabricate novel assemblies. Nanovesicles and nanotubes were successfully constructed by crosslinking cyclo-(DP)3 with hexamethylendiamine or cyclo-RR based on covalent bonding or electrostatic interaction. To further explore its potential application in nucleotide delivery, plasmid DNA and oligoRNA were successfully bound to the vesicles and the latter was taken into the cell. On the basis of this result, more complex functions, such as gene delivery and targeted siRNA delivery, are expected to achieve with these cyclic peptide assemblies.
Video
Video is unavailable in archive mode. Watch on YouTube
Introduction
Over the last several decades, nucleic acid delivery has been extensively used for biomedical purposes since it is the most straightforward way to regulate gene expression and in turn affect cell function. Therapeutics based on this strategies hold tremendous promise for the treatment of some most severe diseases, such as human severe combined immunodeficiency disease (SCID), genetic disorders and cancer1-5. However, despite its promising prospect of curing both inherited and acquired diseases, nucleic acid delivery is still unavailable in clinical use owing to its low efficiency to deliver the nucleic acid to the target sites6. The inefficiency is caused by a series of reasons, including kidney filtration, phagocyte uptake, serum protein adhering and enzymatic degradation3, 7. In addition, since both nucleic acids and cell membranes are negatively charged in physiological conditions, it is hard for nucleic acids to be directly taken in by tissue cells due to charge repulsion7. Thus, the strategies of constructing nucleic acid carriers that are able to overcome these physiological barriers are the key challenges in this field.
Nanotechnology is a great help to improve the delivery efficiency. Taking advantage of their nanoscale sizes, nanoparticles can inherently prolong blood circulation by avoiding the filtration of capillary endothelium and kidney8. And due to the abnormality of diseased regions, nanoparticles can passively accumulate in tumor tissues through enhanced permeability and retention (EPR) effects9. Moreover, nanoparticles can deeply penetrate tumor tissue and exhibit a significantly improved cellular uptake efficiency both in vitro and in vivo11. And the surface of nanoparticles can be easily functionalized to incorporate desired characteristics, such as endosomal escape12 and rapid release of genes and drugs13-14. These inherent priorities make nano-sized carriers the most promising candidate for nucleic delivery.
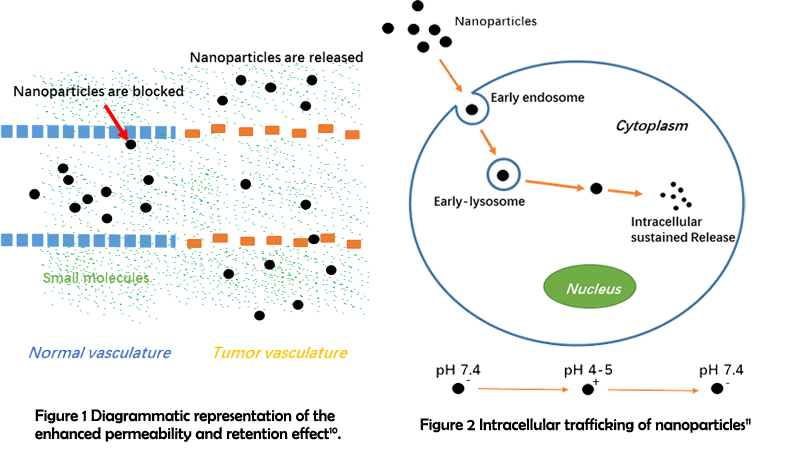
Great efforts have been devoted to constructing nano-carriers for nucleic acid delivery, with a wide range of materials emerging, such as polymers12, porous silica nanoparticles15 and graphene16. However, these materials suffer from either toxicity or poor water solubility17. Therefore, it is particularly important to develop carriers with improved biocompatibility and solubility. Biomolecule-based nanostructures are of great interests in biomedical application due to their rich structural diversity and inherent biocompatibility. Among them, cyclic peptides have received remarkable attention owing to their rigid configuration as well as chemical and enzymatic stability18. To date, previous reports have mainly focused on the construction of nanotubes in a ring-stacking manner through hydrogen bonding19. The possibility of using cyclic peptide side chains to extend the assembling methodology and functions have rarely been explored. This has inspired us to construct novel assemblies based on the sidechain reaction. We expect that our boldly proposed design can contribute to the enrichment of nucleic acid delivery strategies and give some inspirations for controllable nano-synthesis.
REFERENCES
1. Cavazzana-Calvo, M.; Hacein-Bey, S.; Basile, G. d. S.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.-L.; Bousso, P.; Deist, F. L.; Fischer, A., Gene Therapy of Human Severe Combined Immunodeficiency (SCID)-X1 Disease. Science 2000, 288 (5466), 669-672.
2. Nishikawa, M.; Huang, L., Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Human Gene Therapy 2001, 12 (8), 861-870.
3. Kanasty, R.; Dorkin, J. R.; Vegas, A.; Anderson, D., Delivery materials for siRNA therapeutics. Nat Mater 2013, 12 (11), 967-977.
4. Carthew, R. W.; Sontheimer, E. J., Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136 (4), 642-655.
5. Oh, E. J.; Park, K.; Kim, K. S.; Kim, J.; Yang, J.-A.; Kong, J.-H.; Lee, M. Y.; Hoffman, A. S.; Hahn, S. K., Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. Journal of Controlled Release 2010, 141 (1), 2-12.
6. El-Sayed, A.; Futaki, S.; Harashima, H., Delivery of Macromolecules Using Arginine-Rich Cell-Penetrating Peptides: Ways to Overcome Endosomal Entrapment. Aaps Journal 2009, 11 (1), 13-22.
7. Whitehead, K. A.; Langer, R.; Anderson, D. G., Knocking down barriers: advances in siRNA delivery. Nature Reviews Drug Discovery 2009, 8 (2), 129-138.
8. Alexis, F.; Pridgen, E.; Molnar, L. K.; Farokhzad, O. C., Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Molecular Pharmaceutics 2008, 5 (4), 505-515.
9. Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K., Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 2000, 65 (1-2), 271-284.
10. Iyer, A. K.; Khaled, G.; Fang, J.; Maeda, H., Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today 2006, 11 (17�?8), 812-818.
11. Panyam, J.; Labhasetwar, V., Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews 2003, 55 (3), 329-347.
12. Majzoub, R. N.; Ewert, K. K.; Safinya, C. R., Cationic liposome–nucleic acid nanoparticle assemblies with applications in gene delivery and gene silencing. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2016, 374 (2072).
13. Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O. C., Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chemical Reviews 2016, 116 (4), 2602-2663.
14. Karimi, M.; Ghasemi, A.; Zangabad, P. S.; Rahighi, R.; Basri, S. M. M.; Mirshekari, H.; Amiri, M.; Pishabad, Z. S.; Aslani, A.; Bozorgomid, M.; Ghosh, D.; Beyzavi, A.; Vaseghi, A.; Aref, A. R.; Haghani, L.; Bahrami, S.; Hamblin, M. R., Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chemical Society Reviews 2016, 45 (5), 1457-1501.
15. Slowing, II; Vivero-Escoto, J. L.; Wu, C. W.; Lin, V. S. Y., Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced Drug Delivery Reviews 2008, 60 (11), 1278-1288.
16. Yang, K.; Feng, L. Z.; Shi, X. Z.; Liu, Z., Nano-graphene in biomedicine: theranostic applications. Chemical Society Reviews 2013, 42 (2), 530-547.
17. Lewinski, N.; Colvin, V.; Drezek, R., Cytotoxicity of nanoparticles. Small 2008, 4 (1), 26-49.
18. Sun, L. M.; Fan, Z.; Wang, Y. Z.; Huang, Y. J.; Schmidt, M.; Zhang, M. J., Tunable synthesis of self-assembled cyclic peptide nanotubes and nanoparticles. Soft Matter 2015, 11 (19), 3822-3832.
19. Brea, R. J.; Reiriz, C.; Granja, J. R., Towards functional bionanomaterials based on self-assembling cyclic peptide nanotubes. Chemical Society Reviews 2010, 39 (5), 1448-1456.